CNN
—
For a potentially serious medical condition, it’s remarkable how few people know they have obstructive sleep apnea (OSA).
OSA, in which a person’s upper airway collapses, causing irregular breathing, is the most common sleep-related breathing condition. One study estimated well over 900 million adults between 30 and 69 years old may have it worldwide, though as many as four in five moderate-to-severe cases are undiagnosed. Left untreated, it can increase the risk of high blood pressure, cardiovascular disease, hearth attack, stroke, and type 2 diabetes, and yet untreated is what most cases — diagnosed and undiagnosed — are.
The good news is that for people with a diagnosis, there have never been more ways to remedy the problem. With the global sleep apnea device market valued at $8.52 billion in 2024 and projected to rise to nearly $13 billion by 2030, according to one 2025 report, medical tech companies are developing new solutions.
Continuous positive airway pressure (CPAP) machines, which push air into the mouth to maintain open airways, are highly effective and have long been considered the gold standard treatment.
However, CPAP can have a high initial dropout rate, with users citing the discomfort of wearing a mask strapped to the face, or the lifestyle adaptations required to use the machine.
Despite CPAP’s dominance, the marketplace for solutions is opening up. Mandibular advancement devices worn over teeth hold the lower jaw and tongue forward; neurostimulation implants trigger the hypoglossal nerve into action to prevent the tongue and soft tissues from blocking airways; there’s also a drug being trialed that’s designed to stimulate the nerve.
Another alternative is negative pressure devices, which unlike CPAP, suck instead of blow, pulling the tongue and soft tissues up and forward in the mouth. So far, such devices haven’t been shown to be as effective as CPAP, but the technology is slowly finding its place in the market, with Taiwanese company Somnics Health carving out a foothold.
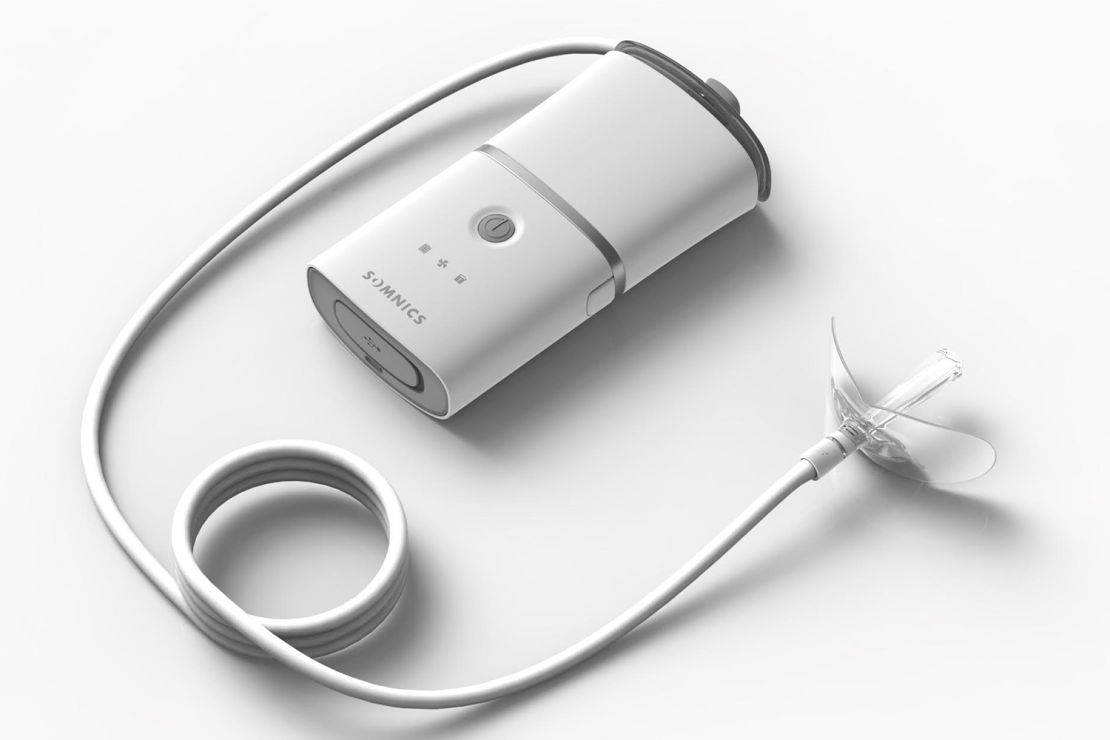
Roughly the size of a smartphone, Somnics’ iNAP is a battery-powered device that attaches to a narrow tube with a flexible mouthpiece at the other end. That is worn inside the mouth, and once switched on, negative pressure keeps airways open. Users then breathe through their nose as normal.
“The beauty of it is it allows natural breathing … and it’s a very comfortable, quiet and efficient solution,” said Somnics general manager Olivier Lauzeral in a video interview.
The iNAP was conceived over a decade ago by Somnics founder Chung Chu Chen, who combined his research with intellectual property purchased from US company Apnicure (which went out of business in 2017, said Lauzeral) to create a negative pressure product. Initial R&D and clinical work took place in Taiwan, where it was first approved by authorities. Since then it has been gradually introduced to new markets across Asia and Europe, winning multiple design awards.
In 2020 it was cleared by the US Food and Drug Administration for use in the United States among adults with obstructive sleep apnea who won’t use CPAP. Today, around 3,000 patients use the iNAP in the US, said Lauzeral, and around 10,000 worldwide.
While studies have shown that negative pressure devices are less effective than CPAP, Lauzeral said that the company’s target market is patients who decide not to use CPAP and mandibular devices, but are not ready for a surgical solution like neurostimulation.
“Seventy percent of our patients are ‘CPAP failing,’” he explained. “That was our first way to adopt patients — to talk to those who had that experience with CPAP. The other 30% are what we call ‘naïve patients,’ (meaning) they hadn’t tried any other treatments before.”
Lauzeral hopes that the convenience of a discreet and portable device that can run for five nights on a single charge will attract patients from younger demographics.
“Half of the population with sleep apnea is younger than 53,” he said. “A lot of those patients don’t want to touch a CPAP.
“What’s sad to see is those people being in denial, not wanting to be treated when they’re in their 30s or early 40s. They don’t get into their treatment, then their sleep apnea deteriorates… 10 years later they have no choice but getting into CPAP and at that point their health is really not good.”

Somnics has recently introduced a subscription model in the US, whereby patients can pay for the device in installments over 24 months, which retails at $1,399.
Unlike most CPAP machines, the prescription-only iNAP is not currently covered by health insurance in the US, though Lauzeral said Somnics is hoping to change this.
Dr. Hrayr Attarian, a sleep medicine specialist and professor of neurology at Northwestern University Feinberg School of Medicine, told CNN that there need to be bigger studies on the efficacy of iNAP.
“The research out there was done in small groups of people,” he said in an email. “Even with those limitations,” studies showed improvements in 75% and 83% of patients, “vs. close to 95% with CPAP,” he added.
Attarian characterized the device as “an alternative but not a replacement for CPAP,” though agreed that Somnics’ treatment was “less intrusive.”
Surveying the market, although “OSA treatments are going to be more fragmented… nothing so far has the same efficacy of CPAP,” he said. To become a viable competitor, negative pressure manufacturers need to conduct clinical trials with “a larger number of participants with all degrees of (OSA) severity,” he added.
Dr. Johan Verbraecken, pulmonologist and medical coordinator at the Sleep Disorders Centre, Antwerp University Hospital, agreed that more research would help establish the effectiveness of the iNAP. Currently, it’s not a first-line treatment, he said — particularly for severe cases of OSA. Verbraecken described it as an “add-on treatment instead of a standalone treatment.”
He said that the iNAP has minimal side effects but argued that to compete with CPAP, the product needs to more effectively reduce patients’ Apnea-Hypopnea Index (AHI) — how often a person’s breathing slows or stops during sleep.
“There has always been an interest in (CPAP) alternatives,” he said. “These numbers grow, given more patients get the diagnosis, while the absolute number of people not tolerant to CPAP is growing accordingly. On the other hand, the dropout rate is lowering, given better CPAP and mask technology.”
Somnics will be banking that there will continue to be a percentage of patients who will remain averse to CPAP. “We’re ready to start working with partners that will help us really get to the next level and grow and capture all those patients,” said Lauzeral.

